A Map of the Psychedelic Landscape.
View the full interactive landscape.
Executive Summary
The psychedelic landscape is growing fast, and many people see only small parts of it. It’s like the story of the blind men and the elephant: each person touches one part but can’t grasp the full animal. Our map clearly shows the entire landscape, revealing gaps and opportunities. With this clear view, stakeholders can see where collaboration or funding is needed most.
Delphi, led by Lia Mix, connects investors, healthcare providers, governments, and the psychedelic community. We offer strategic consulting, education, and support partnerships to build a robust psychedelic ecosystem. This map was developed in cooperation with Blossom, a leading resource for psychedelic research aiming to help psychedelics become approved medicines.
This document is living and continuously updated. We invite collaboration to further explore these insights.
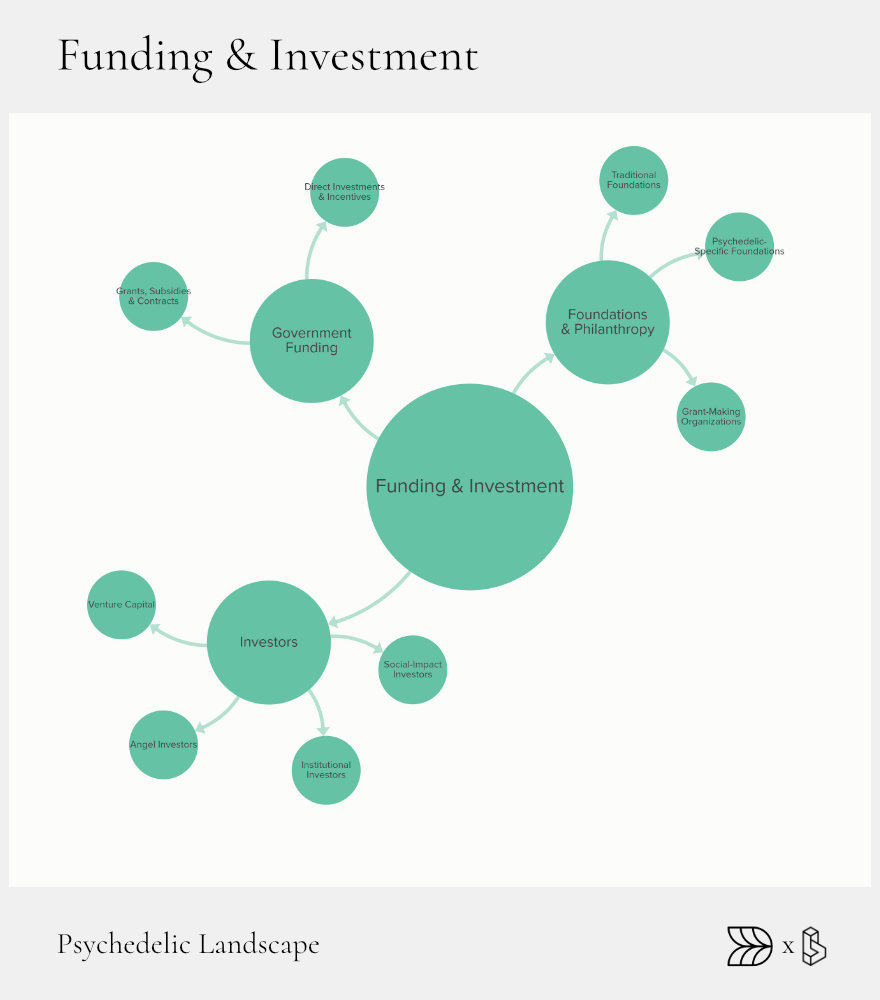
Funding & Investment: The Bedrock
Funding is the foundation of the psychedelic landscape. Without capital, research stalls, clinical trials halt, and promising treatments never reach patients. Over recent years, investor interest has surged. Money flows from individuals, venture capital firms, foundations, and government sources. However, not all funding is created equal, and understanding these differences helps reveal where the gaps are.
The funding landscape has rapidly evolved. Initially driven by philanthropy and angel investors, the field now sees more institutional capital. Yet, investment remains heavily concentrated in North America. Many promising companies still struggle to secure the large sums needed for later-stage trials, causing delays or closures.
Addressing these funding gaps is crucial. Clearly mapping out where investment is most needed can guide capital to underfunded stages and regions, unlocking greater potential across the psychedelic ecosystem.
Investor Archetypes
Investors in psychedelics range from private individuals to specialized venture capital firms. Angel investors often fund early-stage ventures; a notable example is Max Hodak’s $11.5 million investment in Mindstate. Venture capital splits into two main categories: generalist firms expanding into psychedelics and psychedelic-native funds like Palo Santo and Noetic Fund, which have become significant players, collectively deploying millions into the sector.
Strategic corporate venturing is also growing. Pharmaceutical companies like Otsuka have begun acquiring psychedelic-focused startups to expand their portfolios. Meanwhile, social-impact investors and blended-finance groups are emerging, combining financial returns with social goals to create pathways for ethical and equitable development.
Foundations & Philanthropy
Philanthropy remains crucial, particularly for research and early-stage innovation. Legacy health foundations, like the Steven & Alexandra Cohen Foundation, have started funding psychedelic research, signaling mainstream acceptance. Psychedelic-specific foundations such as MAPS and Beckley Foundation continue to play significant roles, funding groundbreaking research and advocacy.
Donor-advised funds (DAFs) and giving circles have also facilitated significant financial support. The Pineapple Fund famously donated $5 million to MAPS via Bitcoin, demonstrating how non-traditional philanthropic vehicles can rapidly mobilize resources.
Government Funding
Government funding provides critical validation and financial stability. Competitive NIH-style grants have begun targeting psychedelic research explicitly. For example, NIDA recently solicited research proposals for psilocybin, LSD, MDMA, and ibogaine to address substance use disorders.
Tax credits and R&D incentives also play a supportive role, enabling companies to offset high research costs. State innovation funds, like Texas’s earmarked $50 million for ibogaine research on opioid use disorder, illustrate how regional governments are stepping ahead of federal agencies to drive innovation.
Current Bottlenecks
Despite these advances, major challenges remain. Late-stage clinical trials require substantial capital, often exceeding $100 million. Sparse funding at this stage has already led companies like Compass Pathways to lay off staff and delay their timelines.
Moreover, funding remains geographically skewed, with more than 80% of investment concentrated in the U.S. Non-dilutive funding, such as grants, remains limited outside North America, making it difficult for international psychedelic research to scale efficiently.
Addressing these funding gaps requires better coordination among investors, philanthropists, and governments. Increased transparency and strategic mapping can direct capital more effectively, supporting innovation through each critical phase of development.
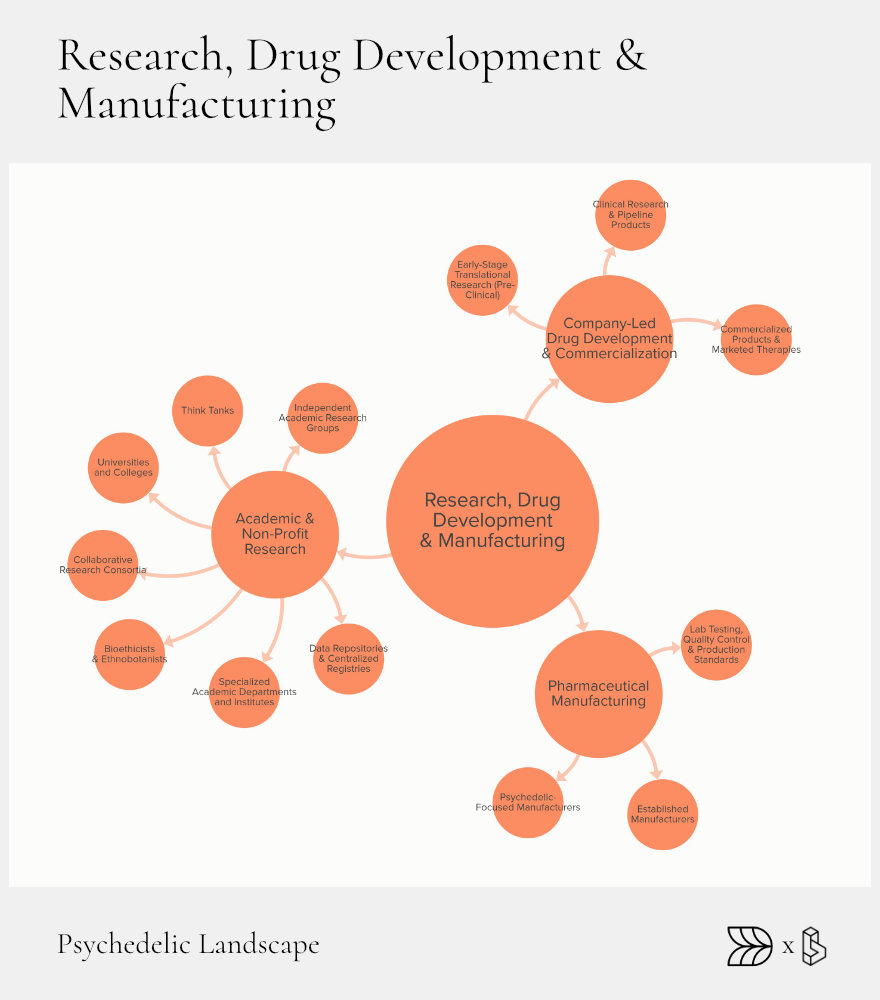
Research, Drug Development & Manufacturing: Pathway to Approval
Research and development form the heart of psychedelic progress. This sector transforms promising ideas into proven treatments through rigorous scientific validation. Universities, non-profits, and private companies each play critical roles, ensuring that potential therapies navigate complex regulatory pathways and reach those who need them.
University-based research centers like Johns Hopkins and Imperial College London have led early research efforts, establishing psychedelic studies as credible science. These institutions have provided foundational insights, from basic neuroscience to groundbreaking clinical outcomes.
Private companies build upon academic research, moving potential therapies through clinical trials and regulatory approval. This collaborative ecosystem relies heavily on efficient, compliant manufacturing practices, ensuring the highest standards of safety and consistency.
Academic & Non-Profit Research
Top academic institutions anchor the psychedelic research landscape. Johns Hopkins, Imperial College, and UC Berkeley operate dedicated centers that publish influential studies and provide public education. Collaborative efforts like data trusts and open-science consortia, such as Therapsil’s real-world data trust in Canada, encourage sharing critical insights to speed up progress.
Company-Led Development
Private companies are critical in translating research into viable medicines. Early-stage innovators, like Delix Therapeutics, develop new compounds, including non-hallucinogenic alternatives. Meanwhile, companies like Compass Pathways and Lykos Therapeutics manage advanced clinical trials, navigating regulatory hurdles for treatments like psilocybin for depression and MDMA for PTSD.
Drug-device innovations are also essential, with companies like Apollo Neuro developing technology to enhance psychedelic therapy effectiveness, helping reduce anxiety and improve patient outcomes during sessions.
Manufacturing & Quality
Reliable manufacturing under strict regulatory standards (cGMP) is essential for therapeutic use. Contract manufacturers like Psygen specialize in psychedelic compound production, providing essential support for research and clinical trials.
Innovative manufacturing methods, such as Octarine Bio’s biosynthetic fermentation processes, represent significant advances. They offer scalable and sustainable production methods that could greatly expand treatment accessibility.
Regulatory Milestones
Regulatory approval remains a pivotal step. The FDA’s acceptance of new drug applications (NDAs) and designation of therapies as Breakthrough Therapies accelerates the review and approval process. Notably, Lykos Therapeutics received priority review for MDMA therapy, highlighting the critical importance of regulatory support in advancing treatments.
Despite progress, clearer pathways and better-supported manufacturing capabilities are needed to ensure therapies move efficiently from laboratories to clinics. Enhanced cooperation among research institutions, private companies, and regulators will be crucial.
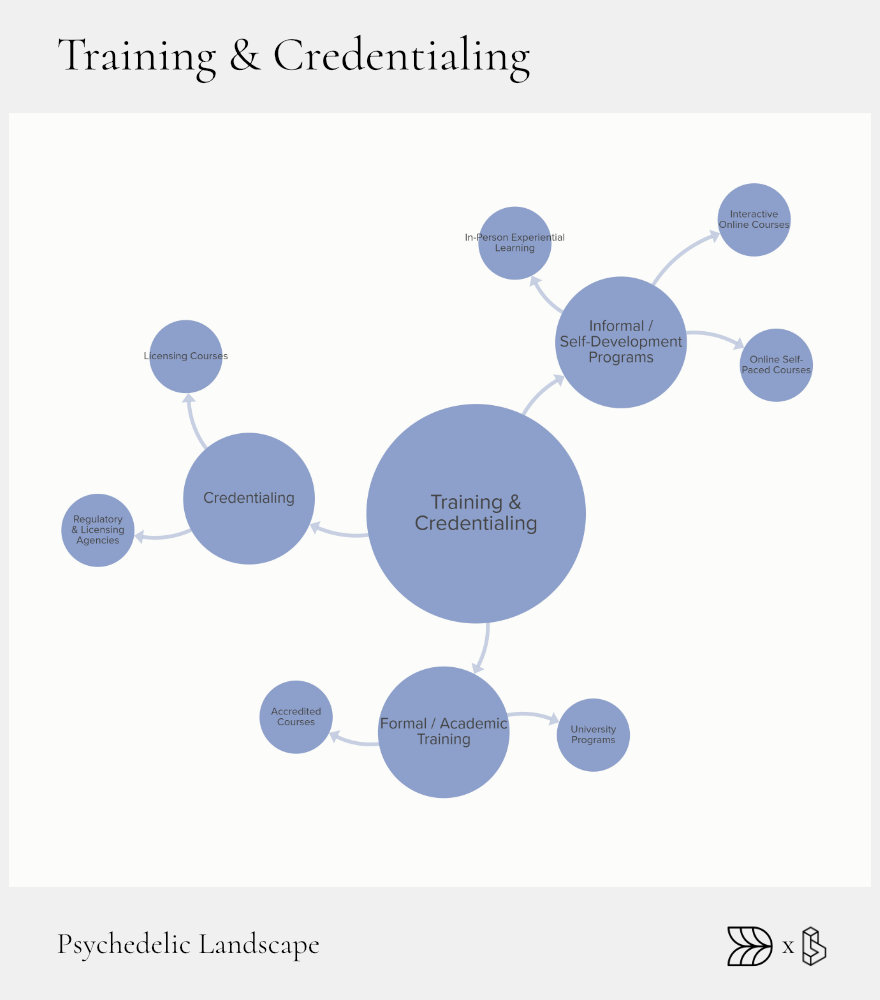
Training & Credentialing: Building Human Capital
Training is critical for safely delivering psychedelic therapies. The growing field needs skilled practitioners who understand both clinical and ethical responsibilities. Without proper training and certification, the promise of psychedelic medicine risks being undermined by unsafe or ineffective practice.
Formal academic programs have emerged to meet this need, with universities incorporating psychedelic studies into graduate-level curricula. These structured programs offer a blend of clinical theory, practical skills, and ethical training. Meanwhile, informal educational programs provide experiential and flexible learning options to reach a broader audience.
Credentialing bodies play an essential role by standardizing training requirements and ensuring practitioner competence. Clear, recognized standards are crucial for the professionalization of psychedelic-assisted therapies, building public trust, and ensuring consistent patient outcomes.
Formal Academic Tracks
Universities now offer specialized degrees and certificates in psychedelic therapies. Programs like Columbia University’s Master’s track in psychedelic therapy and the University of Wisconsin’s graduate courses in psychoactive pharmaceuticals exemplify structured education paths that prepare practitioners thoroughly.
Informal & Experiential Programs
Many practitioners gain experience through shorter, targeted training courses and immersive retreats. Programs like Synthesis Institute’s experiential training offer intensive, hands-on learning that supplements formal academic studies. Online courses and workshops further broaden access, providing flexible, practical education.
Credentialing Bodies
Organizations like Oregon Psilocybin Services set standards for licensing and certification, ensuring practitioners meet rigorous criteria. Voluntary professional boards also support the development of consistent ethical standards and continuing education requirements.
Current Challenges
Despite progress, significant barriers remain. Training can be expensive, limiting diversity and accessibility among practitioners. Additionally, no universally accepted core competencies exist yet, making standardization difficult. Addressing these issues through funding, scholarships, and broader access to training programs will be essential to building a robust workforce.
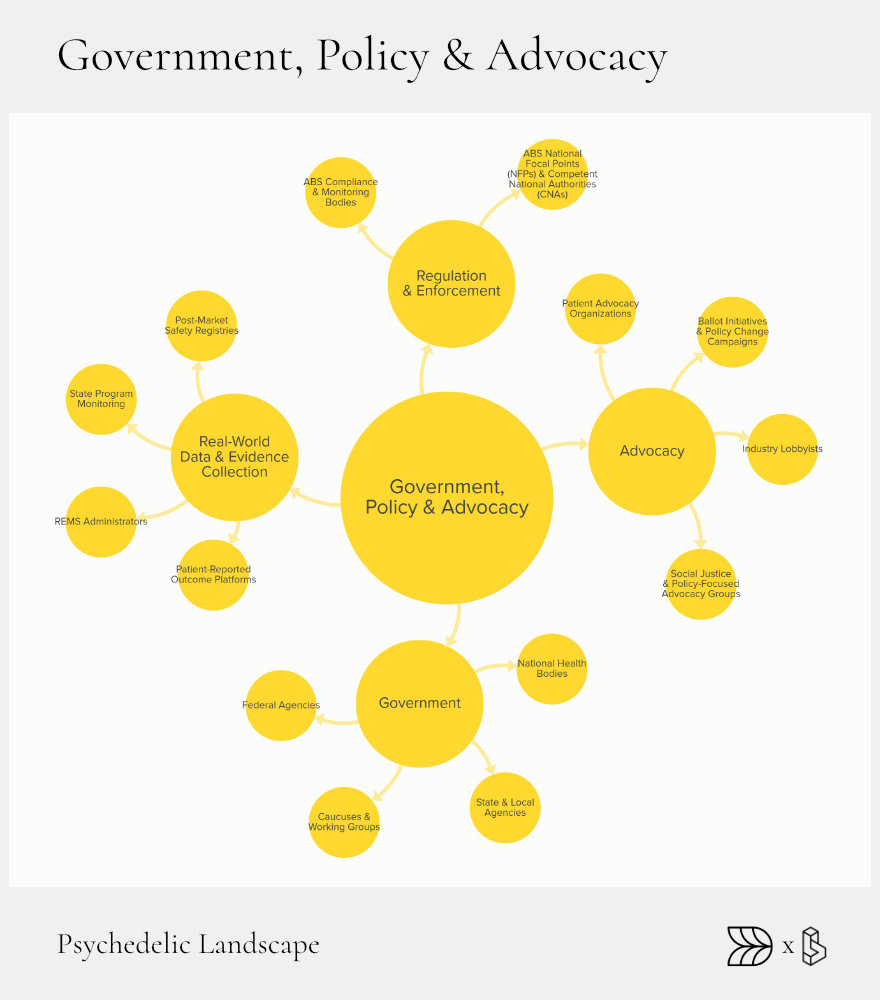
Government, Policy & Advocacy: Setting the Rules
The regulatory landscape shapes how psychedelic treatments move from research to real-world application. Effective policies and advocacy determine how quickly and safely these therapies reach patients. Governments, regulators, and advocacy groups each play distinct but interlinked roles, creating the frameworks necessary for sustainable growth in psychedelic medicine.
Government involvement varies significantly across regions. Some jurisdictions, like Oregon and Colorado in the United States, have developed comprehensive regulatory frameworks for therapeutic use. Federal agencies such as the FDA and EMA set critical guidelines that shape clinical trials, manufacturing, and marketing approval, while international agreements like the Nagoya Protocol manage ethical sourcing and use of genetic resources.
Advocacy groups amplify patient voices, influence legislative changes, and promote public education. These groups drive progress by pushing for policy reforms, increasing accessibility, and ensuring ethical and equitable treatment.
Regulators
Regulatory bodies, including the FDA (U.S.), EMA (EU), and TGA (Australia), are critical gatekeepers in psychedelic medicine. They establish guidelines for research, clinical trials, and therapeutic approval. Recent regulatory shifts, such as the FDA granting Breakthrough Therapy status to MDMA and psilocybin therapies, accelerate the approval process and signal growing institutional acceptance.
State-level initiatives also significantly influence the landscape. Oregon’s Psilocybin Services, for example, created a comprehensive licensing and regulatory system for therapeutic psilocybin use. Similar efforts in Colorado are shaping how future states might manage psychedelic therapies, balancing safety with access.
Legislative & Electoral Fronts
Legislation and ballot initiatives are essential tools for shaping psychedelic policy. The success of initiatives in cities like Denver, which decriminalized psilocybin, set precedents leading to broader state-wide reforms. Legislative caucuses, such as the U.S. Congressional Psychedelics Advancing Therapies (PATH) Caucus, further these efforts by promoting informed policy discussions and proposing supportive legislation.
Advocacy Coalitions
Patient advocacy and industry coalitions actively campaign for reforms that enhance access and equity. Veteran-led groups, patient organizations, and social justice coalitions have effectively mobilized support for initiatives like “Right-to-Try,” enabling early access to psychedelic therapies for terminal or severely ill patients.
These coalitions also advocate for standardized industry practices around labeling, insurance coverage, and patient safety. Their ongoing efforts ensure that psychedelic treatments are not only approved but also accessible and equitable.
Enforcement & Compliance
As psychedelics move toward broader legal acceptance, enforcement and compliance remain critical. Regulatory agencies monitor manufacturing, import/export controls, and distribution channels, maintaining strict adherence to national and international regulations.
Real-world data registries and Risk Evaluation and Mitigation Strategies (REMS) are essential tools used by regulatory bodies to monitor post-approval safety, efficacy, and compliance, protecting public health as new therapies enter the market.
Clear regulatory pathways, collaborative advocacy, and robust compliance frameworks are essential to sustaining progress. Governments, industry groups, and advocates must continuously collaborate to refine policies that safely expand therapeutic access.
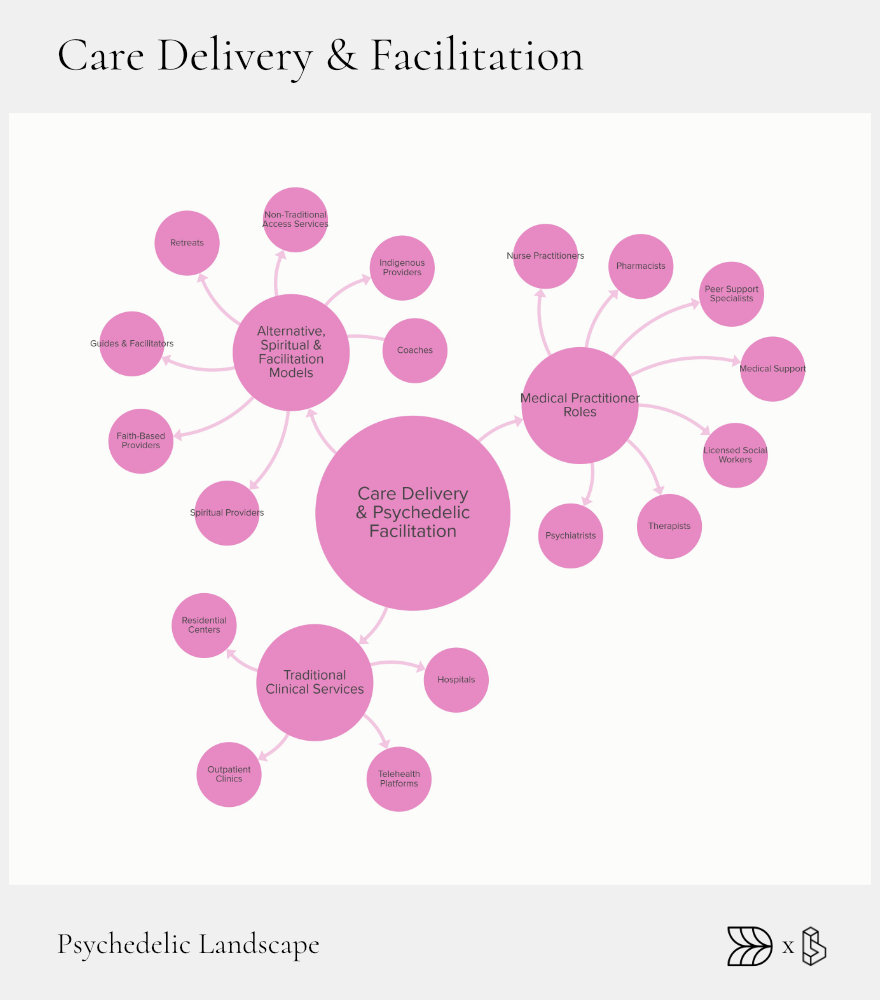
Care Delivery & Facilitation: From Protocol to Patient
Effective care delivery ensures that psychedelic therapies translate from clinical approval into patient healing. Diverse settings, skilled practitioners, and innovative models support patient safety and maximize therapeutic outcomes. As psychedelics move toward broader clinical use, developing robust infrastructure and varied treatment approaches is increasingly critical.
Care delivery encompasses traditional clinical environments, innovative telehealth solutions, and alternative settings rooted in spiritual or community practices. Each model brings unique benefits and addresses different patient needs and preferences. Building a skilled workforce to support these varied approaches is vital for scaling psychedelic therapies effectively.
Integration, the process of helping patients incorporate insights from psychedelic experiences, is equally important. Tools like digital journaling apps, structured integration sessions, and community-based support ensure lasting therapeutic benefits.
Clinical Infrastructure
Clinical settings for psychedelic therapy range from outpatient clinics to specialized hospital units. Outpatient clinics, such as those operated by Field Trip Health and Numinus Wellness, offer structured, supervised sessions in accessible urban locations. Hospital-based programs, though currently limited, provide critical options for complex cases requiring additional medical oversight.
Telehealth platforms, like Mindbloom, have expanded access by providing remote consultation, preparation, and integration support, enabling broader patient reach beyond geographic constraints.
Workforce Mix
A diverse and trained workforce is essential for safe and effective therapy delivery. Psychiatrists, therapists, nurse practitioners, pharmacists, and specially trained facilitators each have defined roles in psychedelic therapy protocols. In jurisdictions like Oregon, distinct licensing frameworks create opportunities for new professional roles tailored explicitly to psychedelic facilitation.
Ensuring adequate training, certification, and continuous education for these practitioners is essential. Standardized credentialing helps maintain high-quality care, patient safety, and therapeutic effectiveness.
Alternative & Spiritual Models
Alongside clinical models, alternative settings like indigenous-led retreats and faith-based organizations offer meaningful therapeutic experiences. Retreats guided by traditional healers, such as those run by Shipibo practitioners in Peru, combine ancestral practices with modern support structures. Similarly, psychedelic churches provide legally protected spiritual spaces for sacramental use, highlighting the diverse ways psychedelics can be integrated into care.
Underground peer-support networks also exist, providing informal guidance and integration services outside formal frameworks, underscoring the need for safe, regulated access to meet patient demand.
Integration Toolkits
Integration support is crucial to psychedelic therapy success. Digital tools, including journaling and self-monitoring apps, help patients process experiences between sessions. Structured integration models, combining individual therapy with group sessions, offer comprehensive emotional and community support, significantly enhancing therapeutic outcomes.
To scale effectively, care delivery systems must address infrastructure gaps, workforce training, and integration practices. Collaboration among clinical providers, alternative practitioners, and technology developers will ensure that psychedelic therapies benefit diverse patient populations safely and effectively.
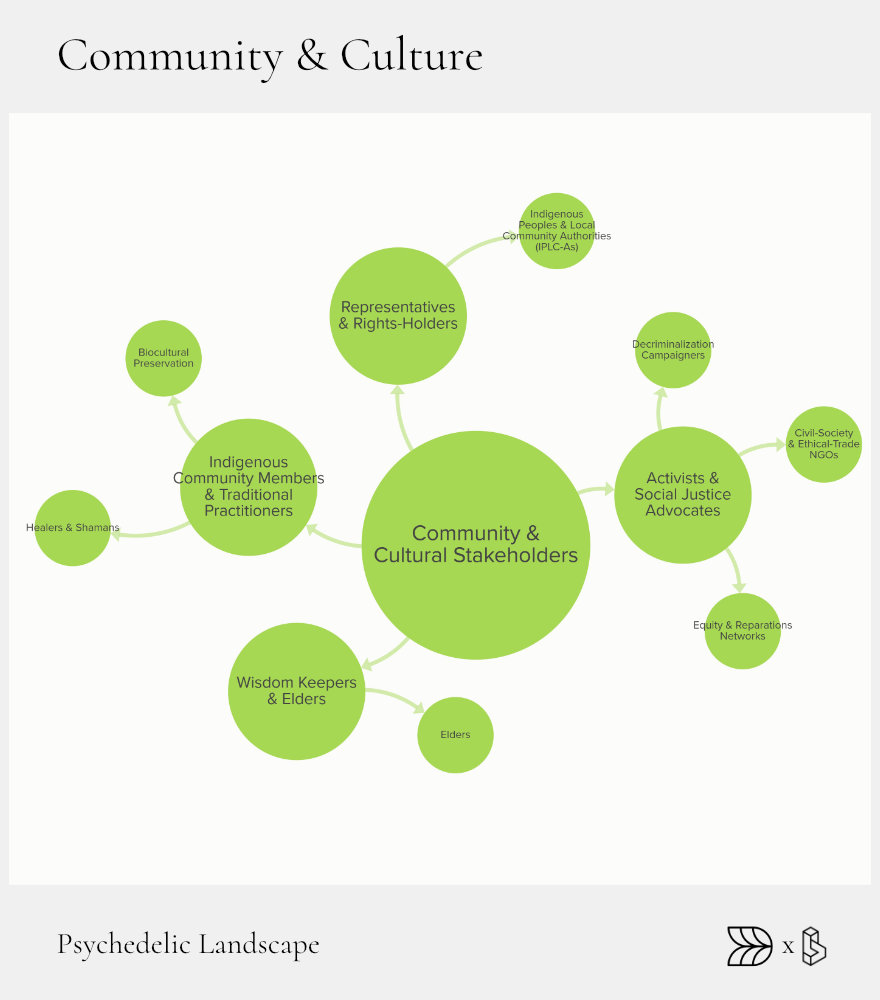
Community & Culture: Grassroots Heartbeat
Community and culture form the social foundation of the psychedelic landscape. This sector represents the grassroots networks, indigenous communities, and activist groups that nurture cultural acceptance, advocate for ethical practices, and ensure equitable access. These stakeholders remind the broader ecosystem of the importance of cultural respect, community support, and historical context.
Indigenous wisdom keepers and cultural elders steward ancient traditions, providing critical context and safeguarding ancestral knowledge. Activist networks mobilize to drive legislative change, protect communities from exploitation, and demand equitable benefit-sharing from the emerging psychedelic economy. Local collectives create safe, supportive environments for education and integration, grounding psychedelic practices in real-world community connections.
A respectful approach to community and culture helps preserve the integrity of traditional practices while integrating psychedelic treatments into mainstream healthcare. Recognizing and supporting these stakeholders ensures sustainable and ethical growth in the psychedelic sector.
Wisdom Keepers & Elders
Indigenous elders and traditional healers, such as Shipibo curanderos in Peru, are crucial guardians of ancestral psychedelic knowledge. Their practices, which include ceremonial and therapeutic uses of plant medicines, provide invaluable insights into holistic healing. Collaboration between traditional practitioners and modern researchers helps integrate valuable traditional knowledge into contemporary therapeutic practices, ensuring cultural continuity and ethical reciprocity.
Activist Networks
Grassroots activism plays a significant role in psychedelic reform, focusing on decriminalization, social justice, and reparative policies. Groups like Decriminalize Nature have successfully advocated for legal reforms across numerous cities, shifting public perceptions and policies toward greater acceptance. Equity-focused initiatives, such as The Sabina Project, ensure the emerging psychedelic industry addresses historical injustices, promoting inclusive and fair practices.
Local Collectives
Local psychedelic societies and meet-up groups foster community connections, education, and mutual support. Organizations like the NYC Psychedelic Society facilitate regular integration circles, educational workshops, and harm reduction activities. These community-led initiatives strengthen the local infrastructure for safe, informed psychedelic practices, ensuring users have access to essential resources and peer support.
Ethical Trade & Biocultural Preservation
Ethical trade and benefit-sharing practices are essential to protecting Indigenous communities and ensuring the sustainable use of traditional resources. Initiatives developing frameworks for Access and Benefit-Sharing (ABS) agreements help ensure that communities benefit fairly from commercialized traditional knowledge. Ethical sourcing practices and community-driven agreements create equitable relationships between industry stakeholders and traditional custodians.
To strengthen community and culture, ongoing collaboration, cultural sensitivity, and active support for grassroots networks are essential. Integrating these stakeholders into broader ecosystem planning ensures ethical growth and cultural integrity.
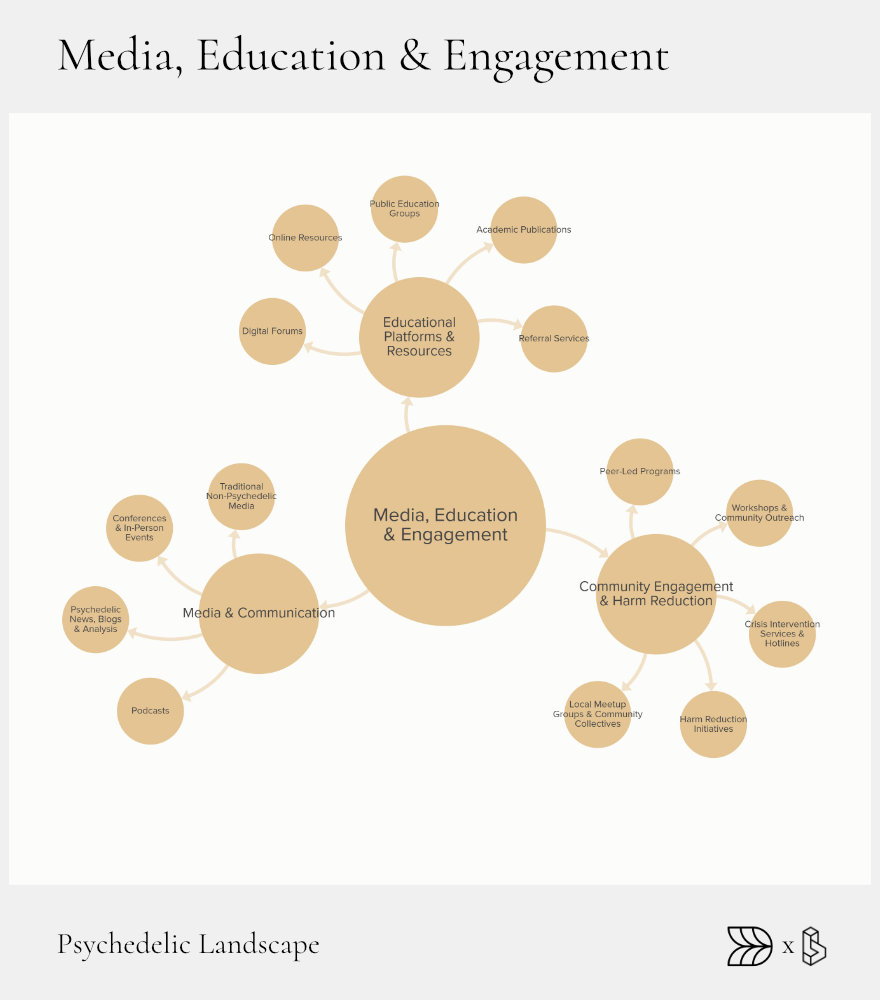
Media, Education & Engagement: Story, Signal, Safety
Effective media, education, and public engagement underpin the responsible growth of the psychedelic ecosystem. Clear, accurate information increases public awareness, ensures patient safety, and builds informed communities. Media outlets, educational platforms, and harm reduction initiatives all contribute significantly to shaping public understanding and acceptance of psychedelic therapies.
Educational platforms range from formal academic programs to accessible online resources, providing comprehensive and trustworthy information. Specialized media channels offer in-depth reporting and analysis, helping stakeholders stay informed about industry trends, clinical developments, and regulatory changes. Harm reduction initiatives deliver crucial services that safeguard individuals and communities, reducing risks associated with psychedelic experiences.
Together, these stakeholders create a balanced, informed public discourse, promote safe use, support effective policy advocacy, and foster widespread cultural acceptance.
Educational Platforms
Online courses and educational hubs, like the Psychedelic Education Center from Psychedelics Today, provide accessible, high-quality learning experiences for diverse audiences. Referral directories, such as Psychedelic Support, offer essential resources connecting patients with qualified therapists and integration specialists, supporting informed, safe therapeutic experiences.
Media Channels
Dedicated media outlets like Psychedelic Alpha and Lucid News offer specialized reporting and insightful analysis on industry developments, clinical research, and policy issues. Podcasts, including Psychedelics Today and the MAPS Podcast, extend public education through engaging discussions with researchers, clinicians, and advocates. Major conferences, such as MAPS’ Psychedelic Science, serve as vital platforms for knowledge-sharing, networking, and collective strategy-building.
Harm-Reduction & Crisis Services
Harm reduction services play a crucial role in minimizing risks and enhancing safety. Organizations like the Fireside Project provide free, confidential peer support lines, offering immediate assistance during challenging psychedelic experiences. Similarly, festival-based harm reduction initiatives like the Zendo Project create safe spaces and deliver peer-support services, significantly reducing risks associated with recreational psychedelic use.
Enhancing media, education, and engagement requires continued investment in accurate reporting, accessible education, and robust harm reduction resources. Strengthening these areas ensures public discourse remains informed, responsible, and supportive of the safe integration of psychedelics into society.
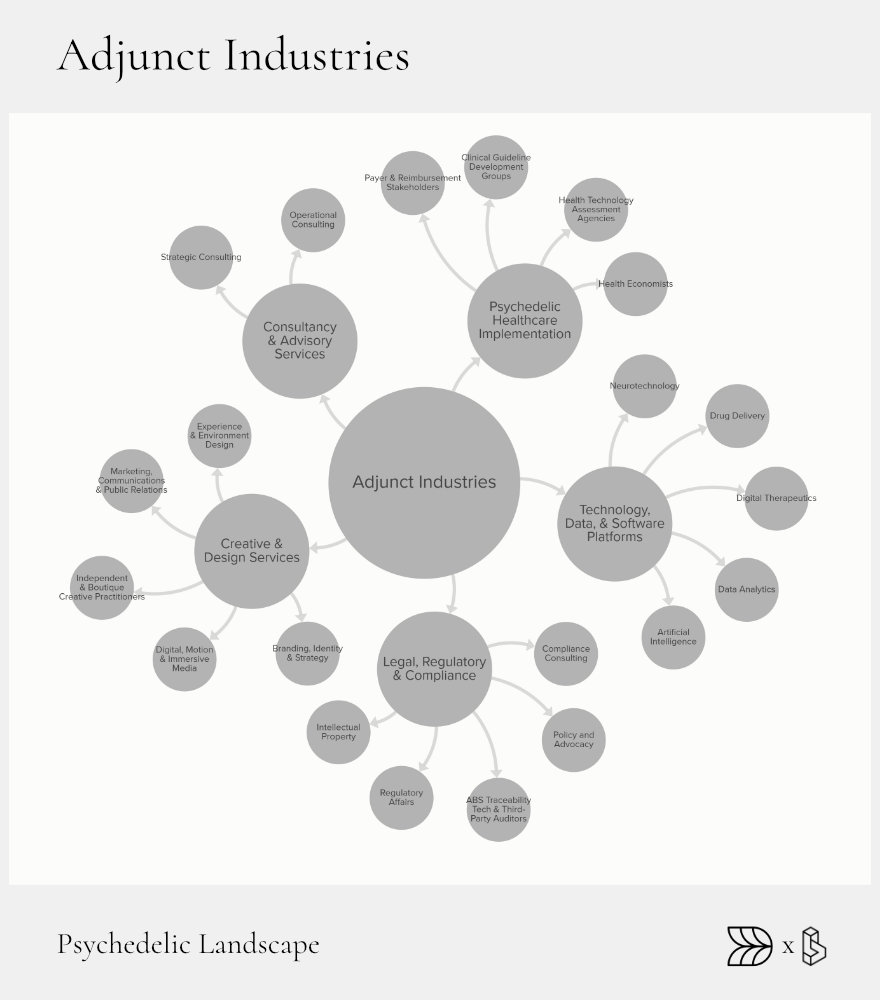
Adjunct Industries: Enablers & Multipliers
Adjunct industries provide essential support services that enable and amplify growth across the psychedelic landscape. These sectors include legal, technological, consultancy, and creative services. Each plays a critical role in helping psychedelic stakeholders navigate regulatory frameworks, develop innovative technologies, and communicate effectively.
Legal and regulatory expertise ensures compliance and smooth operation within complex legal environments. Technological innovation enhances treatment delivery, patient monitoring, and data management, directly improving therapeutic outcomes. Consultancy and creative services shape public perception, brand identity, and operational effectiveness, further supporting the sector’s professional growth.
Collectively, adjunct industries significantly accelerate progress within the psychedelic ecosystem by offering specialized, essential capabilities that stakeholders may lack internally.
Legal, Regulatory & Compliance
Law firms and compliance consultants provide crucial guidance on navigating the evolving regulatory landscape surrounding psychedelics. Specialized firms like Calyx Law and Epstein Becker Green help companies manage complex legal issues around Schedule I substances, intellectual property, clinical trials, and site compliance, enabling safe and legal operational expansion.
Health-Economics & Payer Strategy
Health economics, such as the Collaborative for the Economics of Psychedelics (CEP), assist in demonstrating the clinical and economic value of psychedelic therapies to payers and regulatory bodies. This work helps ensure treatments are not only approved but also covered by insurance, making them financially accessible to patients.
Technology & Software
Technological innovations greatly enhance psychedelic therapy outcomes. Digital therapeutic platforms enable patient tracking, session management, and data analysis to optimize treatments. Neurotechnology wearables such as Apollo Neuro devices provide real-time physiological monitoring and anxiety management, enhancing session safety and efficacy.
Consultancy
Strategic consultancy services, including those provided by firms like Delphi, assist stakeholders in navigating market entry, regulatory hurdles, and operational planning.
Beyond planning, consultancies broker trust. They bring regulators, payers, and community leaders into the same room, so surprises drop. When questions on ethics, supply chains, or reimbursement surface, a seasoned team can solve them fast.
Creative
Creative agencies offer branding, environmental design, and experiential design services. They shape patient experiences and public perceptions, which are vital for patient acceptance and industry growth.
Ongoing investment in these adjunct services is critical to further enabling the sector. Enhanced collaboration and innovation within these industries will ensure comprehensive, robust support for the broader psychedelic landscape.
Appendices
Glossary
ABS (Access and Benefit-Sharing): Ethical framework ensuring equitable sharing of benefits derived from genetic resources.
Angel Investor: Individual investor providing early-stage funding to startups.
Breakthrough Therapy: FDA designation accelerating development and review of promising therapies.
cGMP (Current Good Manufacturing Practice): FDA-regulated manufacturing standards ensuring safety and efficacy.
Clinical Trial Phases (I-IV): Sequential stages of testing a new drug’s safety and efficacy in humans.
Credentialing: The process of certifying a practitioner’s qualifications to deliver psychedelic therapy.
DAF (Donor-Advised Fund): Philanthropic fund allowing donors to manage charitable contributions flexibly.
Decriminalization: Reduction or removal of criminal penalties associated with substances.
Digital Therapeutics: Software-based health interventions complementing traditional therapies.
EMA (European Medicines Agency): European Union agency overseeing pharmaceutical regulation.
FDA (Food and Drug Administration): U.S. agency regulating drug safety, efficacy, and approval.
Harm Reduction: Strategies aimed at minimizing negative consequences associated with substance use.
HEOR (Health Economics and Outcomes Research): Analysis demonstrating economic and clinical value of treatments.
Integration: The process of incorporating insights from psychedelic experiences into everyday life.
MAPS (Multidisciplinary Association for Psychedelic Studies): Non-profit leading psychedelic research and advocacy.
NDA (New Drug Application): Submission to the FDA requesting approval to market a new drug.
NIH (National Institutes of Health): Primary U.S. government agency responsible for biomedical research.
Non-Dilutive Funding: Financial support that doesn’t require equity or ownership stakes.
Payer: Insurance providers and other entities responsible for healthcare payments.
REMS (Risk Evaluation and Mitigation Strategies): FDA-required safety plans for high-risk medications.
Telehealth: Remote healthcare delivery through digital platforms.
TGA (Therapeutic Goods Administration): Australia’s regulatory authority for therapeutic goods, including medications.
Suggested Resources
Pollan, Michael. How to Change Your Mind. Penguin Press, 2018.
Strassman, Rick. The Psychedelic Handbook. Ulysses Press, 2022.
Carhart-Harris, Robin, et al. “Psilocybin with psychological support for treatment-resistant depression” Lancet Psychiatry (2016).
Mitchell, Jennifer, et al. “MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial” Nature Medicine (2023).
Multidisciplinary Association for Psychedelic Studies (MAPS). maps.org.
Psychedelic Alpha. psychedelicalpha.com.
Beckley Foundation. beckleyfoundation.org.
Horizons: Perspectives on Psychedelics Conference. horizons.nyc.
