Reimbursement Pathways for Psychedelic Therapies in Europe
Lia Mix: Welcome to our third Delphi Insight Session. I’m Lia Mix, CEO of Delphi. Today, senior policy and research partner Floris Wolswijk will present on reimbursement pathways in Europe.
Floris Wolswijk: Thanks for joining. I’ll focus on psychedelics and reimbursement in Europe, with lessons that extend to the United States and elsewhere. I’m a senior project manager and researcher at Delphi and a psychologist by training. I started by mapping psychedelic research; clinical trials, outcomes, and what they suggest. I founded Blossom, an intelligence platform tracking the science, and I co‑founded FLO Coaching in the Netherlands, where my wife leads most of the work, helping people with psychedelic-assisted coaching. That model is very different from reimbursed care, and I’ll explain why reimbursement matters. I also co‑authored a report on reimbursement pathways for psychedelic therapies, published in April, with market access consultant Martin Gisby. We examined what implementation might look like across Europe, and how the findings translate to the U.S., Australia, and the U.K.

Why Coverage Defines the Future of Psychedelic Care
Coverage determines whether psychedelic care becomes accessible, trusted, and equitable. I group the “why” into four categories:
- Access and Scale: Today, thousands, at most around 10,000 people in the Netherlands, access psychedelic care through a coaching framework. Oregon, Colorado, and Switzerland reach similarly small numbers. Reimbursement lets us treat thousands, not tens or hundreds.
- Legitimacy and Trust: Moving through Phase I–III trials into authorized use signals quality to people outside the psychedelic ecosystem. Think of those who would never otherwise consider psychedelics. That can reduce stigma.
- Equity: Out‑of‑pocket prices, such as roughly 30,000 Australian dollars, create a two‑tier system. Coverage avoids restricting access to the wealthy.
- Capital Formation: Reimbursed care tells investors this is a legitimate, investable category. We already see interest from pharmaceutical developers.
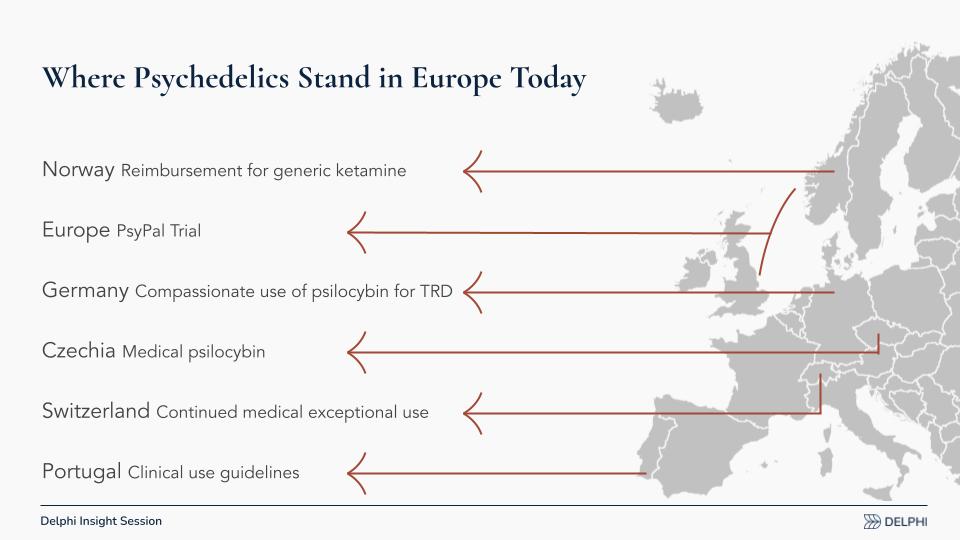
Recent European Momentum and Milestones
Since our April report, Europe has shown fresh momentum toward reimbursed care via several pathways:
- Norway: There is a movement toward reimbursing generic ketamine (as opposed to Spravato, the esketamine [esketamine] nasal spray by Johnson & Johnson). Generic ketamine is far cheaper than branded esketamine; lower prices can expand access but may dampen incentives for developers to pursue authorization at a European scale.
- PsyPal Trial (Europe): A large, multi‑country study in people with life‑threatening illness to address associated anxiety and depression, funded by Horizon [the European Union’s research program]. Crucially, it measures quality of life and healthcare utilization, data European payers weigh heavily.
- Germany: Compassionate use [pre‑approval access for seriously ill patients] of psilocybin for treatment‑resistant depression is slated to begin in select clinics, potentially treating only 50–100 people annually but generating real‑world evidence (RWE).
- Czech Republic: Medical psilocybin is expected next year, with an emerging model and open questions about what will be reimbursed and how. Ketamine is already reimbursable.
- Switzerland: Long‑running, exceptional‑circumstances access to psilocybin, LSD, and MDMA continues for small patient populations, contributing to experience and some data.
- Portugal: New clinical use guidelines are being developed. These are the nuts-and-bolts details of how care should run when treatments become available.
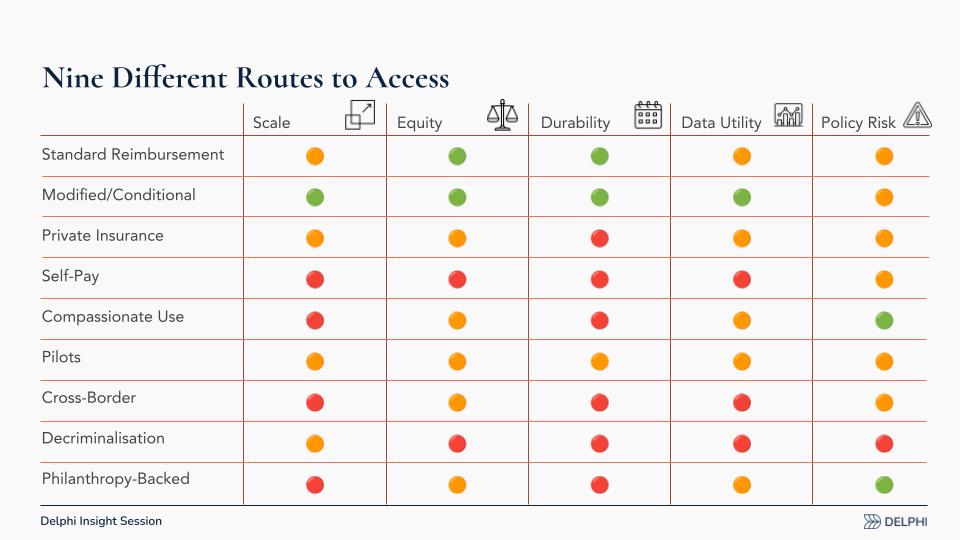
Nine Different Pathways to Access
I’m not focused on “who pays,” but on how to make payment cease to be a barrier. Think about the legal, regulated routes, not decriminalization or unregulated coaching, and assess each against:
- Scale (how many people can be reached)
- Equity (who gets access)
- Durability (can the pathway last)
- Data Utility (does it generate decision‑grade evidence)
- Policy Risk (feasibility in today’s politics)
Self‑pay, compassionate use, cross‑border care, and philanthropic funding can be useful, but they score low on scale, equity, durability, and data utility.
My conclusion: Target a modified or conditional reimbursement pathway rather than only a standard pathway. Psychedelic treatments front‑load cost (preparation, dosing, integration) and may deliver long‑duration benefits. We need to quantify both clinical outcomes and system effects, healthcare utilization and quality‑of‑life impacts, and recognize that countries value “second‑order” benefits (societal effects) differently. Some systems consider them; Germany, for example, tends to focus more narrowly on individual healthcare costs.
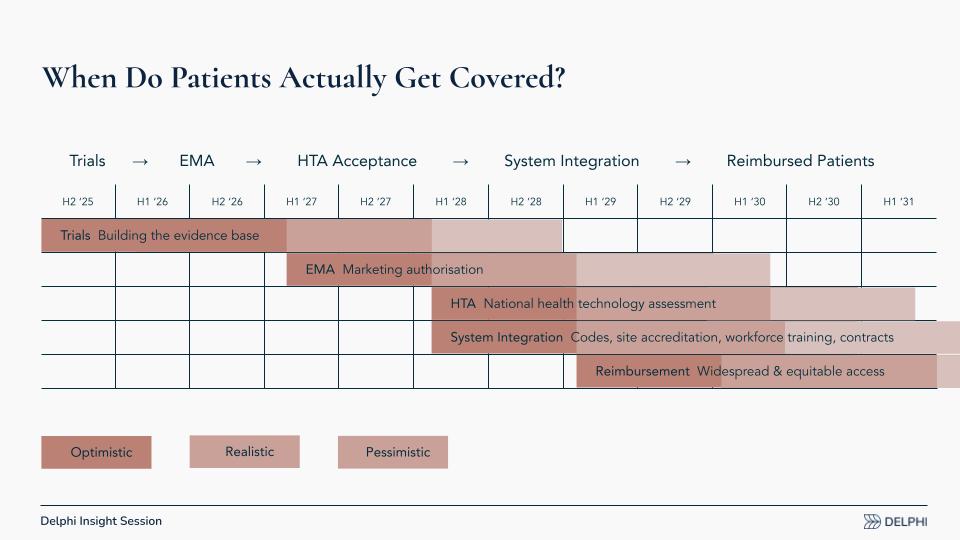
When Do Patients Actually Get Covered?
Coverage in Europe follows a distinct sequence. Timing hinges on when Phase III trials succeed (e.g., programs from Compass, Usona, Lykos, and others) and when developers seek European authorization. The steps:
- Trials and Evidence: Complete Phase III, then submit data.
- EMA Review: The European Medicines Agency (EMA) evaluates efficacy and safety, akin to the U.S. Food and Drug Administration (FDA).
- National HTA Decisions: Each country runs a health technology assessment (HTA) to judge clinical and economic value for that system. Germany often wants head‑to‑head comparisons against the standard of care (e.g., selective serotonin reuptake inhibitors [SSRIs] or specific psychotherapies). A U.S. data package may need supplemental European evidence; some data could be generated post‑authorization, but guidance remains limited.
- System Integration: In parallel, define reimbursement codes, accreditation, and training. Can existing psychologist/psychiatrist/pharmacist codes work, or are new codes needed? Which training programs will professional associations accept, and who must be trained?
- Reimbursement: In an optimistic scenario, early reimbursements could begin around the start of 2029. That may feel distant, but preparation can bring it forward.
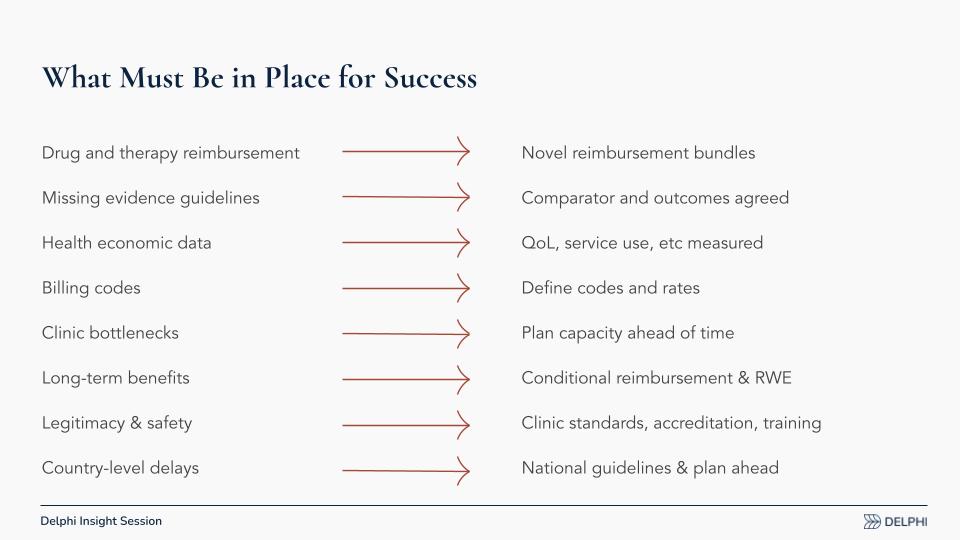
What Must Be in Place for Success
We need to build the plumbing now, shared infrastructure that sits above any single drug or indication, so the system is ready before European Medicines Agency (EMA) filings. If we wait for individual developers to solve this piecemeal, conventions may harden around narrow models that aren’t scalable or equitable. The work splits into data, reimbursement design, and system readiness.
- Data for HTA Decisions: During Phase II and Phase III trials in Europe, capture payer-relevant endpoints, quality of life (QoL), healthcare utilization, and service use, alongside clinical outcomes. Health technology assessment (HTA) bodies need this evidence; U.S.-focused programs may overlook it and treat Europe as an afterthought.
- Agreed Comparators and Outcomes: Align early on the appropriate standard-of-care comparators and decision-grade outcomes. Make those expectations explicit in protocols so European assessors can evaluate psychedelic treatments on terms they recognize.
- Health-Economic Modeling: Europe has very few studies estimating cost-effectiveness for psychedelics. We should analyze available trial data, wherever generated, and map it onto European care pathways and cost structures to create early signals for policymakers.
- Novel Reimbursement Bundles: Design reimbursement that pays for the integrated episode, preparation, dosing, and integration, rather than fragmenting drug and therapy. Bundles should reflect high upfront costs with potential long-duration benefits.
- Billing Codes and Rates: Decide whether existing professional codes suffice or whether new codes are required, and set rates that match the actual work of delivery. This needs agreement across psychologists, psychiatrists, pharmacies, and clinics.
- Capacity and Bottlenecks: Plan clinic capacity ahead of time. Workforce training, scheduling, and site readiness must match likely demand so access is not throttled by logistics.
- Conditional Pathways and RWE: Use conditional or modified reimbursement tied to real-world evidence (RWE) to bridge from trials to broad access while continuing to collect outcomes and utilization data.
- Standards, Accreditation, and Training: Establish clinic standards and accreditation requirements that professional associations will accept. Clarify who must be trained, to what level, and by whom.
- EU-Level Guidance, Local Execution: Develop European-level guidelines and a common blueprint, then localize to each country’s payers, clinical societies, and regulatory context so national adoption is faster and more consistent.
In short, start collecting the right data now, specify how value will be measured, and pre-build the operational rails, codes, rates, capacity, and training so that reimbursement can follow without delay.
Building the Infrastructure Now
One of our active projects at Delphi is developing the system infrastructure that enables equitable, scalable access. We’ve formalised it as RIPE: Reimbursement and Infrastructure for Psychedelics in Europe. Our mission is to build a structured, multi-year roadmap to facilitate tailored reimbursement evaluations and support equitable access for regulatory-approved psychedelic therapies in Europe.
RIPE advances two tracks in parallel:
- Blueprint: Define the concrete work packages: what has to be built, by whom, to what specification, on what timeline, and at what cost. That includes data standards, trial endpoints relevant to HTA, clinic accreditation and training, reimbursement codes and rates, and real-world evidence plans.
- Coalition: Convene stakeholders across Europe (payers, clinicians, professional societies, patient groups, HTA experts, and policymakers) to align on standards, evidence needs, and operational requirements before any single product reaches the EMA stage.
The aim is pre-competitive and pan-European: create shared guidance at the EU level, then localize for each country’s payers and clinical bodies. Rather than leaving system design to the first developer through the gate (or retrofitting later) we can prepare now so access is higher-quality, more efficient, and more equitable from day one. If you’re interested in contributing expertise or funding, please email me at floris.wolswijk@delphi-circle.com and see the RIPE presentation deck below.
Q&A with Lia Mix and Floris Wolswijk
Q1: What Support Does RIPE Need Now?
Lia Mix: Your analysis can accelerate access to psychedelic therapies for more people, sooner. What support do you need to move the RIPE initiative forward so stakeholders align around reimbursement pathways?
Floris Wolswijk: Two tracks.
- Engagement: Stakeholders should engage with European bodies, the European Medicines Agency (EMA), the European Commission, and with national health technology assessment (HTA) systems to align on how to evaluate psychedelic care. We must prepare evaluators for a treatment model with high upfront costs and potential long‑duration benefits compared with talk therapy or selective serotonin reuptake inhibitors (SSRIs). A major payer conference (ISPOR) will soon host its first psychedelics panel. The goal is to bring people outside the psychedelic bubble into the conversation.
- Funding: Philanthropic support is needed for pre‑competitive work developers won’t prioritize, such as system design, shared data standards, and payer‑relevant evidence. If we wait, habits and conventions may harden around a single developer’s model, undermining equity and efficiency.
Q2: What are the Similarities and Differences between U.S. and European Obstacles, Opportunities, and Collaboration?
Lia: Where do Europe and the United States (U.S.) differ, and where can collaboration speed access? Are there opportunities for joint work?
Floris: Europe can borrow lessons from the U.S., especially around billing. Current Procedural Terminology (CPT) codes exist for psychedelic care in the U.S.; that conversation hasn’t started in Europe. We don’t want Europe to passively wait for a U.S. developer to “try Germany and maybe France,” or to rely only on potential mutual agreements with the United Kingdom (UK). We should make the ground more fertile here and also build European capacity. Real‑world evidence (RWE) [data from routine clinical practice outside trials] is a good example: Germany’s compassionate use [pre‑approval patient access pathway] can track depression outcomes, service utilization, costs, and quality of life during and after treatment.
Some are discussing a more speculative route: large‑scale country‑level access (akin to compassionate use) that secures reimbursement without first completing EMA authorization. That could check many boxes and generate data, but it would likely be limited to a single country at the outset.
Lia: When we map tasks across regions, much of the needed work is similar: training standards, clinical guidelines, CPT/billing analogs, and the economic case for coverage. The processes and regulators differ. Europe’s “go slow to go fast” dynamic means once one or two national bodies approve, access can scale broadly across a single‑payer or centralized system. In the U.S., even after Food and Drug Administration (FDA) approval, the multi‑payer coverage process adds years. Shared work products like guidelines, training standards, and economic models should be portable across regions to accelerate adoption and coverage.
Floris: Agreed. In Europe, different “jurisdictions” are countries rather than payers. Smaller countries sometimes follow decisions from larger ones; for example, the Czech Republic may average recommendations from several countries to set reimbursement. Today, HTA is national, but the EU is moving toward a joint HTA framework around 2030. Whether psychedelics will fall under that regime or remain country‑based is still an open question [dependent on how quickly psychedelics move forward].
Q3: Payer Conference Outcomes and Priority Topics
Lia: Kevin Lanzo asks: What would a successful outcome for the psychedelics panel at the payer conference you mentioned be? Which topics should be prioritized now?
Floris: Health economics and outcomes research (HEOR) must become the centerpiece. Europe lacks rigorous, region‑specific HEOR for psychedelic treatments. The first priority involves translating existing trial data to fit European systems by mapping trial outcomes onto European care pathways and cost structures, which would allow stakeholders to estimate budget impact and cost-effectiveness on a country-by-country basis.
Rather than waiting for perfect European datasets to emerge, the approach calls for developing country-level models immediately for top markets using currently available data. These decision-grade models would provide actionable insights even with imperfect information. Recent work, such as Elliott Marseille’s team’s modeling of implementation scenarios in Ukraine, demonstrates effective methods for framing value propositions that could be adapted for the European context through analogous analyses.
To support these efforts, I propose establishing HEOR workstreams under the RIPE framework with dedicated near-term funding. These workstreams would focus on producing concrete, quantifiable metrics that resonate with payers, including quality-adjusted life years, resource utilization data, and calculations of downstream savings. Such hard numbers carry more weight with healthcare decision-makers than narrative arguments alone, making them essential for advancing psychedelic treatments through European regulatory and reimbursement pathways.
Lia: Exactly. Psychedelics are a “spend money to save money” proposition at the societal level. With credible HEOR, we can demonstrate the dollars‑and‑cents rationale for coverage and support higher‑quality care that delivers durable outcomes and, over time, generational benefits.
Floris: Thank you all for being here. Our fourth Insight Session will be next month. It’s always the first Wednesday of the month, so it will be October 1st. We look forward to seeing you then. In the meantime, have a wonderful day. Thanks, everyone.
